The Magnetic Accelerator
e.g. The Gauss Gun
November 3, 2021
The Magnetic Accelerator
e.g. The Gauss Gun
I first saw this demo / activity in 2013 when I enjoyed 3 weeks working with the Energy Project at Seattle Pacific University as an IRISE (Interdisciplinary Research Institute in STEM Education) scholar. The Energy Project’s primary objective was “to promote elementary and secondary teachers’ development of formative assessment practices in the context of energy.” As a scholar in residence, I observed and studied the pedagogical effectiveness of the professional development. And learned a lot of cool stuff about energy!
The energy theater was a major topic, as were energy tracking diagrams as a means to diagrammatically represent and comprehend the energy transfer and transform in different scenarios. These were exciting new representations for me, and they allowed a rich variety of scenarios to be studied, from a melting birdbath, to raising and lowering a bowling ball (more subtle than it sounds!), to switching on a lightbulb.
AND THEN…they pulled out the Gauss Gun. And we, teachers and scholars alike, were all blown away. Figure 1 below shows the basic setup. You get some neodymium magnets, a few ball bearings, and a groove on which the ball bearings can roll. Figure 1 shows the setup on a Pasco optics track, but I’ve used Vernier and Pasco kinematics tracks, and I’ve even seen using the groove in a wooden ruler.

Figure 1: Gauss Gun “before” setup.
In the “before” picture of Fig. 1, three ball bearings (on the right) are magnetically attracted to a neodymium magnet. A single ball bearing (from the left) is given a gentle push toward the magnet. As the single ball approaches the magnet, it feels the magnetic attraction, gets pulled in, and suddenly…the rightmost ball on the opposite side ROCKETS away with unexpected velocity (usually causing first-timers to jump in surprise). Figure 2 shows the “after” picture, with the rightmost ball rolling away.

Figure 2: Gauss Gun “after” result.
At first inspection, you are sure that physics has been broken. Total energy is “clearly” not conserved. The incoming ball had very little kinetic energy and the outgoing ball has A LOT. Of course, physics is not broken, and that’s the point of the activity. To take a surprising and really cool result, perform an energy analysis, and realize that everything works out once you consider the magnetic bonding energy.
Bonding. Now is the moment where we take a neato physics demo and unleash its full power…on chemistry and biology! On the surface, this looks like “bond breaking releases energy,” the common misconception that pervades the biology classroom and drives chemists batty. But to break the bond of the right most ball, you need to give the left ball a little bit of kinetic energy to come in and break the original bond, and the new bond formation (the strong pull of the magnet on the left ball) is what unleashes a large amount of energy. The demo is not a perfect analogy, but it’s pretty good. And it’s fun. Win-win!
Energy spans all scientific disciplines, yet physics, chemistry, and biology often treat the topic from viewpoints that are incompatible. Students, in turn, are unable to apply knowledge earned from one course to another, hampering their ability to make significant conceptual gains. Introductory Physics for the Life Sciences (IPLS) courses are trying to bridge the gap and make connections with many topics in undergraduate chemistry and biology courses. For instance, the IPLS community has identified molecular potential energy, chemical energy, free energy, and entropy as topics in need of dedicated instruction. Much of this initial work was done by the University of Maryland NEXUS project. The Gauss Gun has been identified in the physics community as a model system for exploring exothermic chemical reactions in the context energy conservation, including both qualitative and quantitative investigations.
Below, you can download a guided activity that I wrote for students in my first semester, algebra-based college physics class. I believe it can be readily modified to use in any physics, chemistry, and biology class.
The first two pages are an investigation of the energy scenario discussed in this blog post. Students are guided to consider energy conservation and wrestle with the ideas of bond breaking and bond formation. The third and fourth pages focus on connections with bond enthalpy and coupled reactions in the context of ATP hydrolysis as an exothermic reaction and the first step of cellular respiration as an endothermic reaction. Figure 3 shows an example of a coupled reaction: the initial exchange of energy results in a ball with high kinetic energy that is able to trigger the second endothermic reaction.
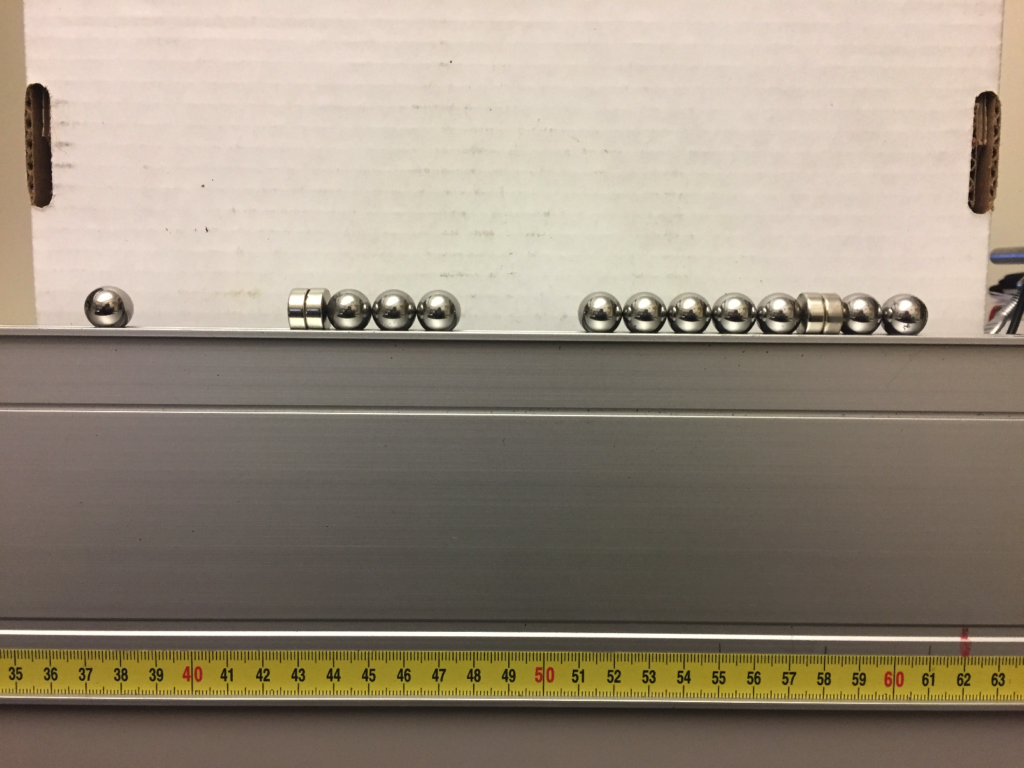
Figure 3: Coupled Reaction
Below are links to UMD’s NEXUS materials about the Gauss Gun, along with some other papers that I’ve read about this activity.
- NEXUS Physics – Gauss Gun qualitative
- NEXUS Physics – Gauss Gun energy bar representation
- Modeling potential energy of the Gaussian gun – 2019 Elliott, et. al.
- Modeling chemical reactions with the Gaussian gun – 2019 Elliot, et. al.
- Chemical energy in an IPLS course – 2014 Dreyfus, et. al.
- Energy and Momentum in the Gauss Accelerator – 2004 Kagan
If you try the activity, let me know how it goes! I’d love to hear feedback, or simply learn if you and/or your students found it valuable.


Recent Comments